Answer:
24.6 mL is the volume of the ball when the temperature spikes 410 K and the pressure increases to 956.2 Torr
Step-by-step explanation:
The combined gas equation is,
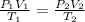
where,
 = initial pressure inside a ball = 879.3 Torr
= initial pressure inside a ball = 879.3 Torr
 = final pressure inside a ball = 956.2 Torr
= final pressure inside a ball = 956.2 Torr
 = initial volume of ball =
= initial volume of ball =
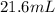
 = final volume of gas = ?
= final volume of gas = ?
 = initial temperature of gas =
= initial temperature of gas =
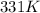
 = final temperature of gas =
= final temperature of gas =
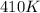
Now put all the given values in the above equation, we get:
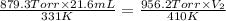
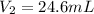
24.6 mL is the volume of the ball when the temperature spikes 410 K and the pressure increases to 956.2 Torr