Answer:
5.8 moles of copper are needed to react with sulfur to produce 2.9 moles of copper (I) sulfide.
Step-by-step explanation:
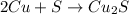
Moles of copper(I) sufide = 2.9 mol
According to reaction, 1 mole of copper(I) sulfide is obtained from 2 moles of copper, then 2.9 moles of copper(I) sulfide will be obtained from :
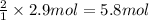 copper
copper
5.8 moles of copper are needed to react with sulfur to produce 2.9 moles of copper (I) sulfide.