Answer:
19.32L
Step-by-step explanation:
Given parameters:
Volume of the gas at STP = 29.3liters;
Using this information first, let us find the number of moles of the gas.
Number of moles =
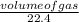
With the number of moles, we can further solve this problem;
Number of moles =
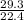 = 1.31 moles
= 1.31 moles
More parameters;
Pressure on gas = 1.39atm
temperature = -23°C = -23 + 273 = 250K
Unknown:
Volume of gas = ?
Solution:
The ideal gas equation is the perfect fix to solve this problem.
It is mathematically expressed as;
Pv = nRT
where P is the pressure
v is the volume
R is the gas constant
n is the number of moles
T is the temperature
Input the parameters;
1.39 x V = 1.31 x 0.082 x 250
V = 19.32L