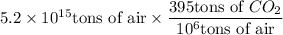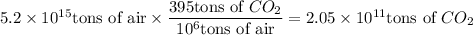Answer:
Step-by-step explanation:
ppm is a unit of concentration that expresses the mass of a component (solute) in 1,000,000 units of mass of a mixture (solution).
Thus, 395 ppm means 395 tons of CO₂ in 1,000,000 tons of air.
To determine the amount of CO₂ in 5.2 × 10¹⁵ tons of air, multiply the amount of air by the ppm concentration of CO₂ divided by 1,000,000 (10⁶):