Answer:
9.7 × 10² g NaF.
Step-by-step explanation:
Molarity is defined by the amount of solute (mols) over the volume of solution (liters):
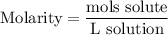
We want to create 6.3 liters of a 3.6 molar solution of NaF.
Solve for the amount of NaF necessary:
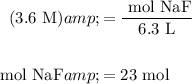
Therefore, about 23 moles of NaF is required.
Convert from moles to grams. The molecular weight of NaF is 41.99 g/mol:
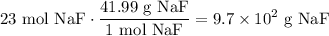
Therefore, about 970 grams of NaF is needed to create the solution.