Answer:
B
Step-by-step explanation:
Recall the law of effusion:
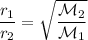
Because 5 mol of oxygen was effused in 10 seconds, the rate is 0.5 mol/s.
Let the rate of oxygen be r₁ and the rate of hydrogen be r₂.
The molecular weight of oxygen gas is 32.00 g/mol and the molecular weight of hydrogen gas is 2.02 g/mol.
Substitute and solve for r₂:
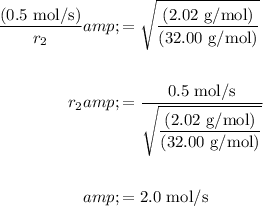
Because there are 5 moles of hydrogen gas:
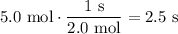
In conclusion, it will take about 2.5 seconds for the hydrogen gas to effuse.
Check: Because hydrogen gas is lighter than oxygen gas, we expect that hydrogen gas will effuse quicker than oxygen gas.