Answer:
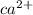
Step-by-step explanation:
In all atoms, the number of protons = number of electrons, as a result the atom is neutral. Losing or gaining electrons will make the atom electrically charged and we call an electrically charged atom an ion.
Ca 2+ would be the symbol because losing two negative electrons makes calcium's nucleus more positive by two protons.