Answer:
208.7°C was the initial temperature of the limestone.
Step-by-step explanation:
Heat lost by limestone will be equal to heat gained by the water
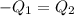
Mass of limestone =
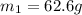
Specific heat capacity of limestone =
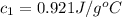
Initial temperature of the limestone =
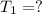
Final temperature =
 =T = 51.9°C
=T = 51.9°C
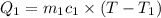
Mass of water=
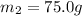
Specific heat capacity of water=
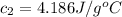
Initial temperature of the water =
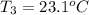
Final temperature of water =
 =T = 51.9°C
=T = 51.9°C
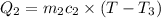
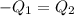
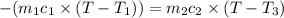
On substituting all values:
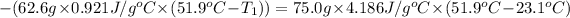
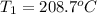
208.7°C was the initial temperature of the limestone.