The answer for the following problem is mentioned below.
- Therefore 148.125 × 10^-23 molecules of the cabon monoxide gas is produced.
Step-by-step explanation:
Given:
mass of the oxygen gas = 395 grams
We know;
For the production of the carbon monoxide;
The equation is,
Before balancing the equation;
C +
 → CO
→ CO
After balancing the equation;
2 C +
 → 2 CO
→ 2 CO
where,
C = carbon molecule
O = oxygen molecule
CO = carbon monoxide
For the equation,
32 grams of oxygen gas → 2 × 6.023 × 10^-23 molecules
395 grams of oxygen gas → ?
=
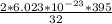
= 148.125 × 10^-23 molecules of the carbon monoxide
Therefore 148.125 × 10^-23 molecules of the cabon monoxide gas is produced.