The answer for the following problem is mentioned below.
- Therefore the pH of an HCl solution is 1.52.
Step-by-step explanation:
pH:
It is a measure of the hydrogen ion concentration,a measure of acidity or the alkalinity of a solution.
(or)
The negative logarithmic function of the hydrogen ion concentration is called the pH.
The formula for the pH is as follows:
pH = -log [
 ]
]
pH less than 7 indicates that solution is acidic
pH greater than 7 indicates that the solution is basic
Given:
hydrogen ion concentration [
 ] = 0.03 = 3 ×
] = 0.03 = 3 ×
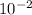
To calculate:
pH of a solution
We know;
pH = -log [
 ]
]
where,
 represents the hydrogen ion concentration
represents the hydrogen ion concentration
pH = -log [3×10^-2]
pH = 1.52
Therefore the pH of a solution is 1.52
Therefore the pH of an HCl solution is 1.52.