Answer:
17.18 moles of NaCl are in 2,719 mL of a 6.32 M solution.
Step-by-step explanation:
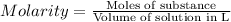
We have:
Molarity of the NaCl solution = 6.32 M
Volume of the NaCl solution = 2,719 mL =2,719 × 0.001 L= 2.719 L
1 mL = 0.001 L
Let the moles of NaCl be n.
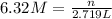
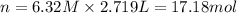
17.18 moles of NaCl are in 2,719 mL of a 6.32 M solution.