Answer:
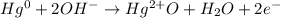
Step-by-step explanation:
Hello,
In this case, mercury (II) oxide (HgO) is obtained via the reaction:
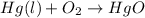
Nonetheless, since it is a reaction carried out in basic solution, mercury's half-reaction only, must be:
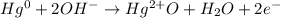
Thus, it is seen that OH ionis should be added due to the basic aqueous solution considering that 2 electrons are transferred from 0 to 2 in mercury.
Best regards.