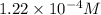This is an incomplete question, here is a complete question.
The generic metal hydroxide M(OH)₂ has Ksp = 7.25 × 10⁻¹². (NOTE: In this particular problem, because of the magnitude of the Ksp and the stoichiometry of the compound, the contribution of OH⁻ from water can be ignored. However, this may not always be the case.)
What is the solubility of M(OH)₂ in pure water? Express your answer with the appropriate units.
Answer : The solubility of M(OH)₂ in pure water is,
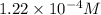
Explanation :
The equilibrium chemical reaction will be:
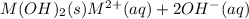
The solubility constant expression for this reaction is:
![K_(sp)=[M^(2+)][OH^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/ctwqec203r8l6uvvbi02tcriq8t83re5r7.png)
Let the solubility be, 'x'
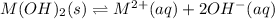
x x 2x
Now put all the given values in this expression, we get:

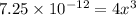
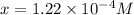
Thus, the solubility of M(OH)₂ in pure water is,