Answer:
The final volume is
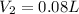 .
.
Step-by-step explanation:
The expression in terms of volume and pressure is as follows;

Here,
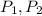 are the initial pressure and the final pressure and
are the initial pressure and the final pressure and
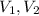 are the initial volume and the final volume.
are the initial volume and the final volume.
This expression is known as Boyle's law.
It is given in the problem that a gas has a volume of 3.0 l at a pressure of 3 kpa. The pressure is increased to 110 kpa.
Calculate the volume,
 .
.

Put
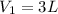 ,
,
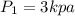 and
and
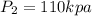 .
.
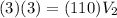
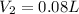
Therefore, the final volume is
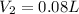 .
.