Answer: The pH of the solution is 12.9
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
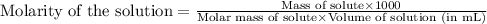
Given mass of KOH = 697 mg = 0.697 g (Conversion factor: 1 g = 1000 mg)
Molar mass of KOH = 56 g/mol
Volume of solution = 160 mL
Putting values in above equation, we get:
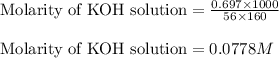
1 mole of KOH produces 1 mole of
 ions and 1 mole of
ions and 1 mole of
 ions
ions
To calculate the pOH of the solution, we use the equation:
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
We are given:
![[OH^-]=0.0778M](https://img.qammunity.org/2021/formulas/chemistry/middle-school/az1zaoic8a6nzpt47r377wql7o148o39p8.png)
Putting values in above equation, we get:
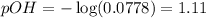
To calculate pH of the solution, we use the equation:
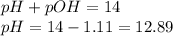
Hence, the pH of the solution is 12.9