Answer:
Cesium has smallest ionization energy.
Step-by-step explanation:
Ionization energy is the energy that an atom at ground state must be absorb to release an electron to form a cation. for eg.
H ⇒
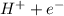
The unit of ionization energy is
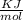 .
.
The ionization energy is minimum for cesium & maximum for fluorine.
Ionization energy depends upon the radius of atom. cesium has smallest radius so it has low Ionization energy.
Therefore the cesium has smallest ionization energy.