32.02 grams/mole is the molar mass of the gas.
Step-by-step explanation:
Data given about the gas:
sample of the gas = 64 grams
volume of the gas = 37.6 L
temperature of the gas = 275K
pressure of the gas = 1.2 atm
R = 0.0821 atm l/mole K
molar mass=?
The data provided will be used to know the molar mass by using ideal gas law equation:
PV = nRT (n =

putting the values in the equation:
n =
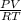
Putting the value of n and then writing the equation:
 =
=
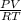
putting the values in the equation
molar mass =
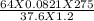
= 32.02 grams/mole