Answer : The precipitate is, AgCl.
Explanation :
The balanced chemical reaction will be:
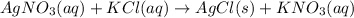
First we have to calculate the molarity of
 ion.
ion.

where,
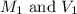 are the initial molarity and volume of KCl.
are the initial molarity and volume of KCl.
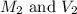 are the final molarity and volume of
are the final molarity and volume of
 ion.
ion.
We are given:
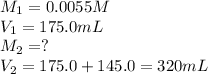
Putting values in above equation, we get:
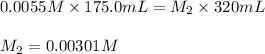
Now we have to calculate the molarity of
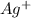 ion.
ion.

where,
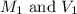 are the initial molarity and volume of
are the initial molarity and volume of
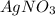 .
.
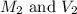 are the final molarity and volume of
are the final molarity and volume of
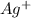 ion.
ion.
We are given:
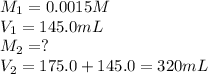
Putting values in above equation, we get:
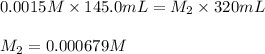
Now we have to calculate the value of reaction quotient.
The expression for reaction quotient will be :
![Q_(sp)=[Ag^+][Cl^-]](https://img.qammunity.org/2021/formulas/chemistry/college/wrg0201c34xui7xrlcx1bvo62c5cr91lv7.png)
Now put all the given values in this expression, we get
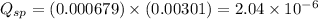
The given solubility constant value is,
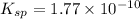
- When
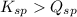 ; the reaction is product favored. (No precipitation)
; the reaction is product favored. (No precipitation) - When
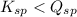 ; the reaction is reactant favored. (Precipitation)
; the reaction is reactant favored. (Precipitation) - When
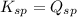 ; the reaction is in equilibrium. (Sparingly soluble)
; the reaction is in equilibrium. (Sparingly soluble)
As,
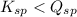 then the reaction is reactant favored that means formation of precipitation.
then the reaction is reactant favored that means formation of precipitation.
Thus, the precipitate is, AgCl.