Answer:
Hence , Ki = Kf
The gas is obeying the Boyle's law.
Step-by-step explanation:
Given data:
The initial volume of the cylinder(in litres) =
 = 12.0 L
= 12.0 L
The initial pressure(in atmospheric pressure) =
 = 4.0 atm
= 4.0 atm
The final pressure(in atmospheric pressure) =
 = 8.0 atm
= 8.0 atm
The final volume of the cylinder(in litres) =
 = 6.0 L
= 6.0 L
First you need to know what Boyle's law is:
Boyle's law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
The Mathematical form of Boyle's law is:
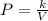
Where,
P = Pressure
V = Volume of the gas
k = Boyle's constant
According to the Boyle's law,
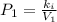
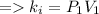
Plug-in the values in the above equation, you would get:
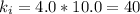
The final pressure(in atmospheric pressure) = = 8.0 atm
The final volume of the cylinder(in litres) == 5.0 L
The Boyle's constant =
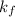 = ?
= ?
According to the Boyle's law,
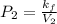
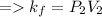
Plug-in the values in the above equation, you would get:

in order to verify Boyle's law, the initial Boyle's constant should be EQUAL to the final Boyle's constant, meaning:
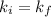
Since,
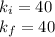
Therefore,
40 = 40
Hence , Ki = Kf
The gas is obeying the Boyle's law.