This is an incomplete question, here is a complete question.
Calculate the free energy ΔG at 25 °C for the nonstandard conditions at point B where the reaction quotient Q is 2.75 × 10⁻⁵.
In solving Part A, we found that ΔG = -40.82 kJ/mol
Answer : The value of
 is, -66.8 kJ/mol
is, -66.8 kJ/mol
Explanation :
The expression for free energy is:
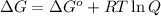
where,
 = free energy = ?
= free energy = ?
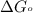 = standard Gibbs free energy = -40.82 kJ/mol
= standard Gibbs free energy = -40.82 kJ/mol
R = gas constant =
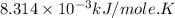
T = temperature = 298 K
Q = equilibrium constant = 2.75 × 10⁻⁵
Now put all the given values in the above formula, we get:
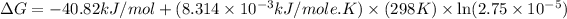
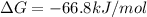
Therefore, the value of
 is, -66.8 kJ/mol
is, -66.8 kJ/mol