Answer:
See the steps below (8,681.25 g of
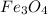 will be needed0
will be needed0
Step-by-step explanation:
From the equation, 4 moles of H2 requires 1 mole of Fe3O4.
1st step: calculate the number of mole of 300 g of H2.
mole = mass/molar mass
Mole of 300 g H2 = 300/2 = 150 mole
Hence, 150 moles of H2 will require: 150/4 = 37.5 moles of Fe3O4.
Step 2: Use the mole of Fe3O4 to calculate the mass of Fe3O4
mass = mole x molar mass
mass of 37.5 mole Fe3O4 = 37.5 x 231.5 = 8,681.25 g
Hence, 8,681.25 g of Fe3O4 will be required to completely react with 300 g H2