Answer:
The total equilibrium pressure of the system if the volume of the reaction vessel is reduced to 1.0 L at constant temperature = 2.4 atm
Step-by-step explanation:
Total pressure of the system (P₁) = 1.2 atm at equilibrium
Volume (V₁) = 2.0 lit
If the volume of the reaction vessel is reduced to (V₂)= 1.0 lit
Pressure (P₂) ?
At constant temperature using Boyle's law
p₁v₁ = p₂v₂
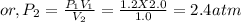
Total pressure at equilibrium is equal to 2.4 atm.