Answer:
The specific gravity of the gel is 1470.68
Step-by-step explanation:
Given:
Volume
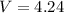

Diameter of tank
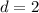 m
m
Pressure at surface
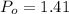 atm
atm
Height
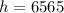 ft
ft
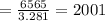 m
m
Pressure at bottom is given by,
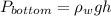
Where
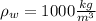 ,
,
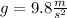
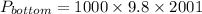
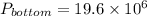
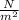
Here volume is 4.24

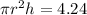
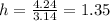 m
m
From pascal equation,
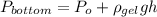
Find density of gel from above equation,
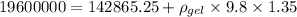
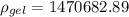
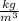
So specific gravity is given by,
ζ =
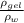
ζ =
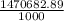
ζ =
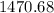
Therefore, the specific gravity of the gel is 1470.68