Given question is incomplete. The complete question is as follows.
A piston-cylinder device initially contains water liquid-vapor mixture at the pressure of 700 kPa. The liquid-vapor mixture has 0.1 m3 of liquid water and 0.9 m3 of water vapor. Heat is transferred at constant pressure until the temperature reaches 320 °C.
(a) What is the initial temperature of the water.
(b) Determine the total mass of the water.
Step-by-step explanation:
(a) We are given that initially there are two phase regions present.
Initial temperature = saturation temperature at 700 Kpa
=
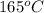
Hence, the initial temperature of the water is
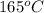 .
.
(b) At 700 Kpa, the saturation pressure will be as follows.
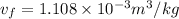
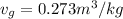
So,
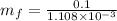
= 90.25 kg
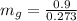
= 3.3 kg
Therefore, total mass will be calculated as follows.
90.25 kg + 3.3 kg
= 93.55 kg
Hence, total mass of the water is 93.55 kg.