Answer:
26.7% is the percent composition by mass of sulfur in a compound named magnesium sulfate.
Step-by-step explanation:
Molar mass of compound = 120 g/mol
Number of sulfur atom = 1
Atomic mass of sulfur = 32 g/mol
Percentage of element in compound :
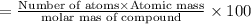
Sulfur :
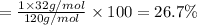
26.7% is the percent composition by mass of sulfur in a compound named magnesium sulfate.