Answer:
The value of partial pressure of oxygen
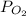 = 83.66 K pa
= 83.66 K pa
Step-by-step explanation:
Volume of oxygen = 80 liters
Temperature = 50°c
The total pressure = 96 K pa
The partial pressure of water at 50°c is calculated from the tables.
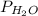 = 12.344 K pa
= 12.344 K pa
The total pressure is given by
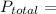
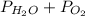
Put all the values in the above equation we get
96 = 12.344 +
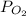
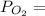 96 - 12.344
96 - 12.344
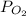 = 83.66 K pa
= 83.66 K pa
This is the value of partial pressure of oxygen.