Answer:
- Letter (f) represents the heat of reaction of both the reverse and the forward reaction.
Step-by-step explanation:
The heat of a reaction, ∆Hrxn, is equal to the potential energy of the products less the potential energy of the reactants:
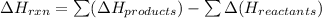
The forward reaction is the conversion of the substances, X and Y, which are represented on the left side of the graph, into the product Z, represented on the right side of the graph.
Thus, for the forward reaction, the change in enthalpy, or ΔH rxn, is the arrow labeled (f):
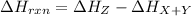
For the reverse reaction, the reactant is Z and the products are X and Y. Thus, heat of reaction for the reverse reaction is:
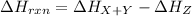
That is the same heat of reaction for the forward reaction with the sign changed: one is positive and the other is negative.
In this case, since the potential energy of Z (on the right side of the graph) is higher than the potential energy of X + Y (on the left side of the graph) the heat of reaction for the forward reaction is positive, the reaction absorbs energy, meaning that it is endothermic.
Therefore, the heat of reaction of the reverse reaction is negative, the reaction releases heat, and is exothermic.
Both heats of reaction are represented by the same arrow labeled (f).