Answer:
1255.4L
Step-by-step explanation:
Given parameters:
P₁ = 928kpa
T₁ = 129°C
V₁ = 569L
P₂ = 319kpa
T₂ = 32°C
Unknown:
V₂ = ?
Solution:
The combined gas law application to this problem can help us solve it. It is mathematically expressed as;
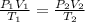
P, V and T are pressure, volume and temperature
where 1 and 2 are initial and final states.
Now,
take the units to the appropriate ones;
kpa to atm, °C to K
P₂ = 319kpa in atm gives 3.15atm
P₁ = 928kpa gives 9.16atm
T₂ = 32°C gives 273 + 32 = 305K
T₁ = 129°C gives 129 + 273 = 402K
Input the values in the equation and solve for V₂;
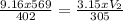
V₂ = 1255.4L