Answer:
Step-by-step explanation:
To solve this problem, we need to obtain the number of moles of the solute we desired to prepare;
Number of moles = molarity x volume
Parameters given;
volume of solution = 500mL = 0.5L
molarity of solution = 0.5M
Number of moles = 0.5 x 0.5 = 0.25moles
Now to know the volume stock to take;
Volume of stock =
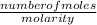
molarity of stock = 4M
volume =
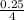 = 0.0625L or 62.5mL
= 0.0625L or 62.5mL