Answer : The value of
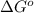 for the reaction is, -5386.4 kJ
for the reaction is, -5386.4 kJ
Explanation :
The given chemical reaction is:
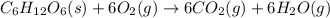
First we have to calculate the entropy of reaction
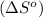 .
.
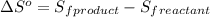
![\Delta S^o=[n_(CO_2(g))* \Delta S^0_((CO_2(g)))+n_(H_2O(g))* \Delta S^0_((H_2O(g)))]-[n_{C_6H_(12)O_6(s)}* \Delta S^0_{(C_6H_(12)O_6(s))}+n_(O_2(g))* \Delta S^0_((O_2(g)))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/q08x2vd69li2b8i27y2vyjrqxqwqf8vm8e.png)
where,
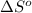 = entropy of reaction = ?
= entropy of reaction = ?
n = number of moles
Now put all the given values in this expression, we get:
![\Delta S^o=[6mole* (211J/K.mol)+6mole* (188.7J/K.mol]-[1mole* (218J/K.mol)+6mole* (206J/K.mol]](https://img.qammunity.org/2021/formulas/chemistry/high-school/7an4kpdg7oargdpc0coctmvruyv5cfitx6.png)
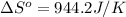
Now we have to calculate the enthalpy of reaction
 .
.
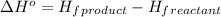
![\Delta H^o=[n_(CO_2(g))* \Delta H^0_((CO_2(g)))+n_(H_2O(g))* \Delta H^0_((H_2O(g)))]-[n_{C_6H_(12)O_6(s)}* \Delta H^0_{(C_6H_(12)O_6(s))}+n_(O_2(g))* \Delta H^0_((O_2(g)))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/3ouovfuil2iwft4evym0w8cqb4ha16ptue.png)
where,
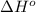 = enthalpy of reaction = ?
= enthalpy of reaction = ?
n = number of moles
Now put all the given values in this expression, we get:
![\Delta H^o=[6mole* (-396kJ/mol)+6mole* (-242kJ/mol]-[1mole* (-1277kJ/mol)+6mole* (0kJ/mol]](https://img.qammunity.org/2021/formulas/chemistry/high-school/jt5a49w92fy603ncivyzwg3jqccrxi2kkf.png)
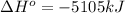
Now we have to calculate the Gibbs free energy.
As we know that,
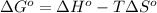
where,
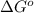 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?
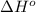 = standard enthalpy = -5105 kJ
= standard enthalpy = -5105 kJ
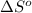 = standard entropy = 944.2 J/K = 0.9442 kJ/K
= standard entropy = 944.2 J/K = 0.9442 kJ/K
T = temperature of reaction = 298 K
Now put all the given values in the above formula, we get:
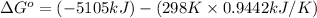
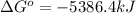
Therefore, the value of
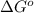 for the reaction is, -5386.4 kJ
for the reaction is, -5386.4 kJ