The given question is incomplete. The complete question is :
The thermal decomposition of phosphine (PH3) into phosphorus and molecular hydrogen is a first-order reaction:
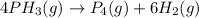 The half-life of the reaction is 35.0 s at 680°C. Calculate the first order rate constant.
The half-life of the reaction is 35.0 s at 680°C. Calculate the first order rate constant.
Answer: a) The first order rate constant is
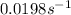
b) The time after which 95% reactions gets completed is 151 seconds
Step-by-step explanation:
Expression for rate law for first order kinetics is given by:
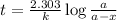
where,
k = rate constant
t = age of sample
a = let initial amount of the reactant
a - x = amount left after decay process
a) for finding the rate constant
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
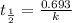
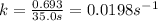
The first order rate constant is
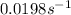
b) for completion of 95 % of reaction
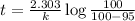
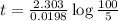
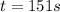
The time after which 95% reactions gets completed is 151 seconds