Answer: Option (A) is the correct answer.
Step-by-step explanation:
A non-metal is a substance which tends to gain electrons from another atom in order to acquire noble gas electronic configuration.
For example, elements of group 6A have 6 valence electrons and elements of group 7A have 7 valence electrons.
Hence, in order to know the charge acquired by the elements of these groups we should subtract their group number from 8.
So, elements of group 6A forms (8 - 6) = 2. That is
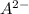 ion is formed in general.
ion is formed in general.
Similarly, elements of group 7A forms (8 - 7) = 1. That is
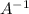 ion is formed in general.
ion is formed in general.
Thus, we can conclude that the ions formed by non-metals in group 6A and 7A have a numerical charge that is found by subtracting the group number from 8.