Answer: The pH of the solution is 9.33
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
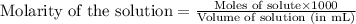 .....(1)
.....(1)
Molarity of HCl solution = 0.200 M
Volume of solution = 7.5 mL
Putting values in equation 1, we get:
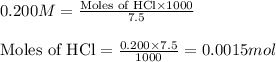
Molarity of trimethylamine solution = 0.100 M
Volume of solution = 20 mL
Putting values in equation 1, we get:
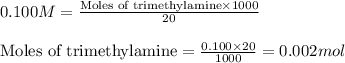
The chemical reaction for trimethylamine and HCl follows the equation:
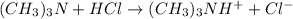
Initial: 0.002 0.0015
Final: 0.0005 - 0.0015
Total volume of the solution = [20 + 7.5] = 27.5 mL = 0.0275 L (Conversion factor: 1 L = 1000 mL)
To calculate the pH of basic buffer, we use the equation given by Henderson Hasselbalch:
![pOH=pK_b+\log(\frac{[\text{conjugate acid}]}{[\text{base}]})](https://img.qammunity.org/2021/formulas/chemistry/college/s1fth0dy4ipogjbdv3s0vtl2jft03r18z2.png)
![pOH=pK_b+\log(([(CH_3)_3NH^+])/([(CH_3)_3N]))](https://img.qammunity.org/2021/formulas/chemistry/college/kr4751hd284cyau4giu98ezpgcr3kfphyd.png)
where,
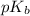 = negative logarithm of base dissociation constant of trimethylamine = 4.19
= negative logarithm of base dissociation constant of trimethylamine = 4.19
![[(CH_3)_3NH^+]=(0.0015)/(0.0275)](https://img.qammunity.org/2021/formulas/chemistry/college/thra5pw6qa5gk6ok3bfy1ri4mgh7a9f1eo.png)
![[(CH_3)_3NH]=(0.0005)/(0.0275)](https://img.qammunity.org/2021/formulas/chemistry/college/hdl9i9zl6clca1o8vt5wdjeiect4gpjfmu.png)
pOH = ?
Putting values in above equation, we get:
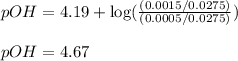
To calculate pH of the solution, we use the equation:
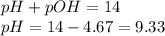
Hence, the pH of the solution is 9.33