Answer:
Because it has equal number of electrons and protons
Step-by-step explanation:
There are three types of particles in an atom:
- Proton: the protons are in the nucleus. They have positive charge equal to
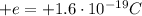 , and mass of
, and mass of
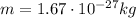 .
.
- Neutron: the neutrons are in the nucleus as well. They have no electric charge and their mass is similar to that of the protons, just slightly smaller.
- Electron: the electrons orbit around the nucleus. They have negative charge opposite to that of the protons,
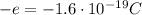 , and they also have much smaller mass (1800 times lighter than the proton). The electrons reside outside the nucleus, in a region of space called "electron cloud". In Bohr's model of the atom, their motion is described as a circular motion, in fixed orbits around the nucleus, where each orbit is associated to a specific energy level.
, and they also have much smaller mass (1800 times lighter than the proton). The electrons reside outside the nucleus, in a region of space called "electron cloud". In Bohr's model of the atom, their motion is described as a circular motion, in fixed orbits around the nucleus, where each orbit is associated to a specific energy level.
Atoms have a neutral charge because they have same number of protons and electrons: therefore, since their charge is the same, the charges from the protons and the electrons cancel out, and the net total charge is zero.