Answer:
The partial pressure of chlorine gas in the mixture is 1.55 atm.
Step-by-step explanation:
Partial pressure of oxygen gas =
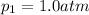
Partial pressure of nitrogen gas =
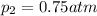
Partial pressure of chlorine gas =
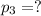
Total pressure of the mixture of gases = P = 3.30 atm
Using Dalton's law of partial pressure:
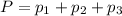
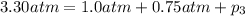
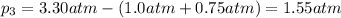
The partial pressure of chlorine gas in the mixture is 1.55 atm.