Answer:
The final volume of the balloon is = 28.11 L
Step-by-step explanation:
Initial pressure
 = 1.03 atm = 104.325 K pa
= 1.03 atm = 104.325 K pa
Initial temperature
 = 26 °c = 299 K
= 26 °c = 299 K
Initial volume
 = 22.4 L
= 22.4 L
Final temperature
 = 22 °c = 295 K
= 22 °c = 295 K
Final pressure
 = 0.81 atm = 82 K pa
= 0.81 atm = 82 K pa
We know that
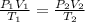
Put all the values in above formula we get
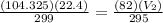
 = 28.11 L
= 28.11 L
This is the final volume of the balloon.