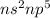Answer:
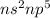
Step-by-step explanation:
Halogen group:This group contains Cl,F,Br and I
There are 7 electrons in the outermost shell of each element of halogen family.
Each element of this family required one electron to fill their complete outermost shell.
We know that
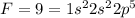
Cl=17=
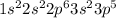
Therefore, the general configuration for atoms of the halogen group is given by