Answer:
The percentage yield of water is 66.67%.
Step-by-step explanation:
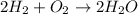
Mass of oxygen gas = 100 g
Moles of oxygen gas =
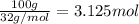
According to reaction, 1 mole of oxygen gives 2 moles of water, then 3.125 moles of oxygen will give:
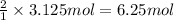
Mass of 6.25 moles of water :
6.25 mol × 18 g/mol = 112.5 g
Theoretical yield of water = 112.5 g
Experimental yield of water = 75 g
Percentage yield :
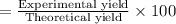
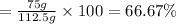
The percentage yield of water is 66.67%.