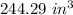Answer:
Explanation:
We apply Boyle's Law that states that pressure and volume for a fixed mass of an ideal gas are exhibit inverse proportionality.
-This law is expressed as:
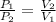
where the a the gas' pressure and volume are varied to assess the change in the other variable.
-Volume 2 is therefore calculated as:
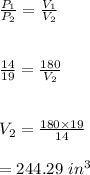
Hence, the volume if the pressure is increased to 19 pounds per square inch is