Answer:
The expression of an equilibrium constant will given as:
![K_c=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/high-school/z3lujc7laty27c6802dkvwnv6vbrp9f6u4.png)
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
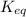
K is the constant of a certain reaction when it is in equilibrium
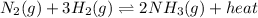
The expression of an equilibrium constant will given as:
![K_c=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/high-school/z3lujc7laty27c6802dkvwnv6vbrp9f6u4.png)