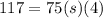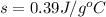Answer:
Specific heat capacity of the copper is given as
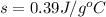
Step-by-step explanation:
As we know that the heat required to raise the temperature of the given substance is given as
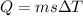
here we know that
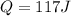
mass of the given part is
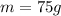
change in temperature due to heat supply is
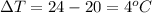
now we have