Answer: The activation energy for backward reaction is +25 kJ
Step-by-step explanation:
For the given chemical equation:
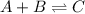
We are given:
Enthalpy of the reaction = -20 kJ
As, the enthalpy of the reaction is negative, the reaction is said to be exothermic in nature.
To calculate the activation energy for the reverse reaction, we use the equation:
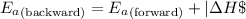
where,
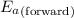 = Activation energy of the forward reaction = 5 kJ
= Activation energy of the forward reaction = 5 kJ
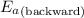 = Activation energy of the backward reaction = ?
= Activation energy of the backward reaction = ?
 = Enthalpy of the reaction = -20 kJ
= Enthalpy of the reaction = -20 kJ
Putting values in above equation, we get:
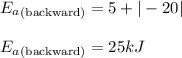
Hence, the activation energy for backward reaction is +25 kJ