Answer:
1.67 m is the molality of a solution .
The boiling point of a solution is 100.85°C.
The freezing point of a solution is -3.1°C.
Step-by-step explanation:
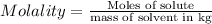
Moles of sugar = 1.25 mol
Mass of water = 0.750 kg
Molality of the solution ;
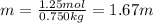
1.67 m is the molality of a solution .
Freezing point of the resulting solution
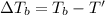
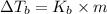
where,
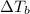 =elevation in boiling point =
=elevation in boiling point =
T' = Boiling point of pure solvent
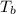 = boiling point of solution
= boiling point of solution
 = boiling point constant
= boiling point constant
m = molality
we have :
 of water = 0.512 °C/m ,
of water = 0.512 °C/m ,
m = 1.67 m
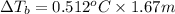
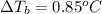
Boiling point of pure water = T' = 100°C
Boiling point of solution =
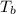
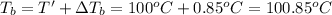
The boiling point of a solution is 100.85°C.
Freezing point of the resulting solution
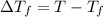
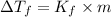
where,
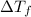 =depression in freezing point =
=depression in freezing point =
T = Freezing point of pure solvent
 = Freezing point of solution
= Freezing point of solution
 = freezing point constant
= freezing point constant
m = molality
we have :
 of water = 1.86°C/m ,
of water = 1.86°C/m ,
m = 1.67 m
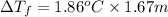
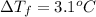
Freezing point of pure water = T = 0°C
Freezing point of solution =

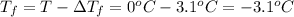
The freezing point of a solution is -3.1°C.