Answer:
Final volume is equal to 0.52 L
Step-by-step explanation:
We have given initially the volume is equal to
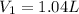
Let the initial pressure is

At constant temperature
 , here
, here
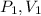 is initial pressure and initial volume respectively and
is initial pressure and initial volume respectively and
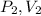 is final pressure and final volume respectively
is final pressure and final volume respectively
Now pressure is doubled so final pressure

So
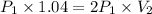
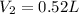
So final volume is equal to 0.52 L