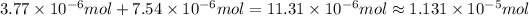Answer:
Total moles of ions are released in water are
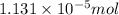 .
.
Step-by-step explanation:
Mass of calcium nitrate =
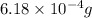
Molar mass of calcium nitrate = 164 g/mol
Moles of calcium nitrate =
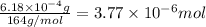
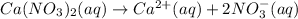
According to reaction, 1 mole of calcium nitrate gives 1 mole of calcium ions, then
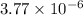 moles of calcium nitrate will give:
moles of calcium nitrate will give:
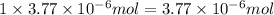 of calcium ions
of calcium ions
According to reaction, 1 mole of calcium nitrate gives 2 mole of nitrate ions, then
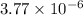 moles of calcium nitrate will give:
moles of calcium nitrate will give:
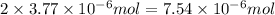 of nitrate ions
of nitrate ions
Total moles of ions are released :