Answer:
Hence, correct answer is option A.
Step-by-step explanation:
Mass of calcium carbonate = 3.25 g
Moles of calcium carbonate =
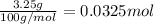
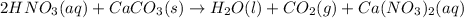
According to reaction, 1 mole of calcium carbonate reacts with 2 moles of nitric acid then 0.0325 moles of calcium carbonate will react with :
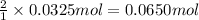
Moles of nitric acid = 0.0650 mol
Volume of the nitric acid solution = V
Molarity of the nitric solution = 0.300 M
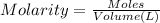
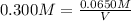
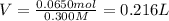
1 L = 1000 mL
0.216 L = 0.216 × 1000 mL = 216 mL
Hence, correct answer is option A.