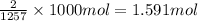Answer:
Energy content per mol fuel 1257 kJ.
Energy content per gram fuel = 27.33 kJ
Energy released per mol carbon dioxide gas formed is 628.5 kJ
Energy released per mol oxygen gas consumed= 419 kJ
Moles of carbon dioxide gas formed per 1000 kJ energy released is 1.591.
Step-by-step explanation:
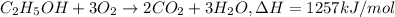
1) Energy released per mole or fuel that is ethanol :
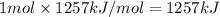
2) Mass of 1 mole of ethanol = 46 g
46 grams of ethanol produces 1257 kJ of energy
Energy content per gram fuel :
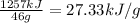
3) Energy released when 2 moles of carbon dioxide are formed = 1257 kJ
Energy released per mol of carbon dioxide formed:
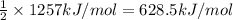
4) Energy released when 3 moles of oxygen gas are consumed = 1257 kJ
Energy released per mol of oxygen gas consumed:
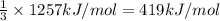
5) Energy released when 2 moles of carbon dioxide are formed = 1257 kJ
Moles of carbon dioxide gas formed per kilo Joule of energy:
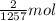
Moles of carbon dioxide gas formed when 1000 kJ of heat is released: