Answer: The concentration of sulfate ions in the solution is 0.010 M
Step-by-step explanation:
The chemical formula of sulfuric acid solution is

We are given:
Concentration of sulfuric acid = 0.010 M
The chemical equation for the ionization of sulfuric acid follows:
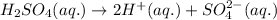
1 mole of sulfuric acid produces 2 moles of hydrogen ions and 1 mole of sulfate ions
Concentration of sulfate ions = (1 × 0.010) = 0.010 M
Hence, the concentration of sulfate ions in the solution is 0.010 M