The answer fr the following problem is mentioned below.
- Therefore no of molecules present are 3.79449 × 10^23 molecules
Step-by-step explanation:
Given:
no of moles (n) = 0.63 moles
We know;
no.of molecules at STP condition (
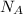 )= 6.023 ×10^23
)= 6.023 ×10^23
To solve:
no of molecules(N)
We know;
to calculate no of molecules present,the formula is mentioned below as follows:
From the ideal gas equation,
n = N ÷
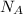
where,
n = no of moles
N = no of molecules
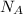 = no of molecules at STP conditions(Avogadro number)= 6.023 ×10^23 molecules
= no of molecules at STP conditions(Avogadro number)= 6.023 ×10^23 molecules
0.63 = N ÷ 6.023 ×10^23
N = 0.63 × 6.023 × 10^23
N = 3.79449 × 10^23 molecules
Therefore no of molecules present are 3.79449 × 10^23 molecules