The answer for the following problem is mentioned below.
Therefore volume occupied by methane gas is 184.78 × 10^-3 liters
Step-by-step explanation:
Given:
mass of methane(
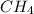 ) = 272 grams
) = 272 grams
pressure (P) = 250 k Pa =250×10^3 Pa
temperature(t) = 54°C =54 + 273 = 327 K
Also given:
R = 8.31JK-1 mol-1 ,
Molar mass of methane(
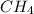 ) = 16.0 grams
) = 16.0 grams
We know;
According to the ideal gas equation,
P × V = n × R × T
here,
n = m÷M
n =272 ÷ 16
n = 17 moles
Therefore,
250×10^3 × V = 17 × 8.31 × 327
V = ( 17 × 8.31 × 327 ) ÷ ( 250×10^3 )
V = 184.78 × 10^-3 liters
Therefore volume occupied by methane gas is 184.78 × 10^-3 liters